How we work?
What started as a small idea has grown into a powerhouse of creativity and innovation.
Clear, concise, and professional communication to ensure project alignment and success.
Demonstrated regulatory pathway expertise, design control mastery, risk management capability, and relevant device experience.
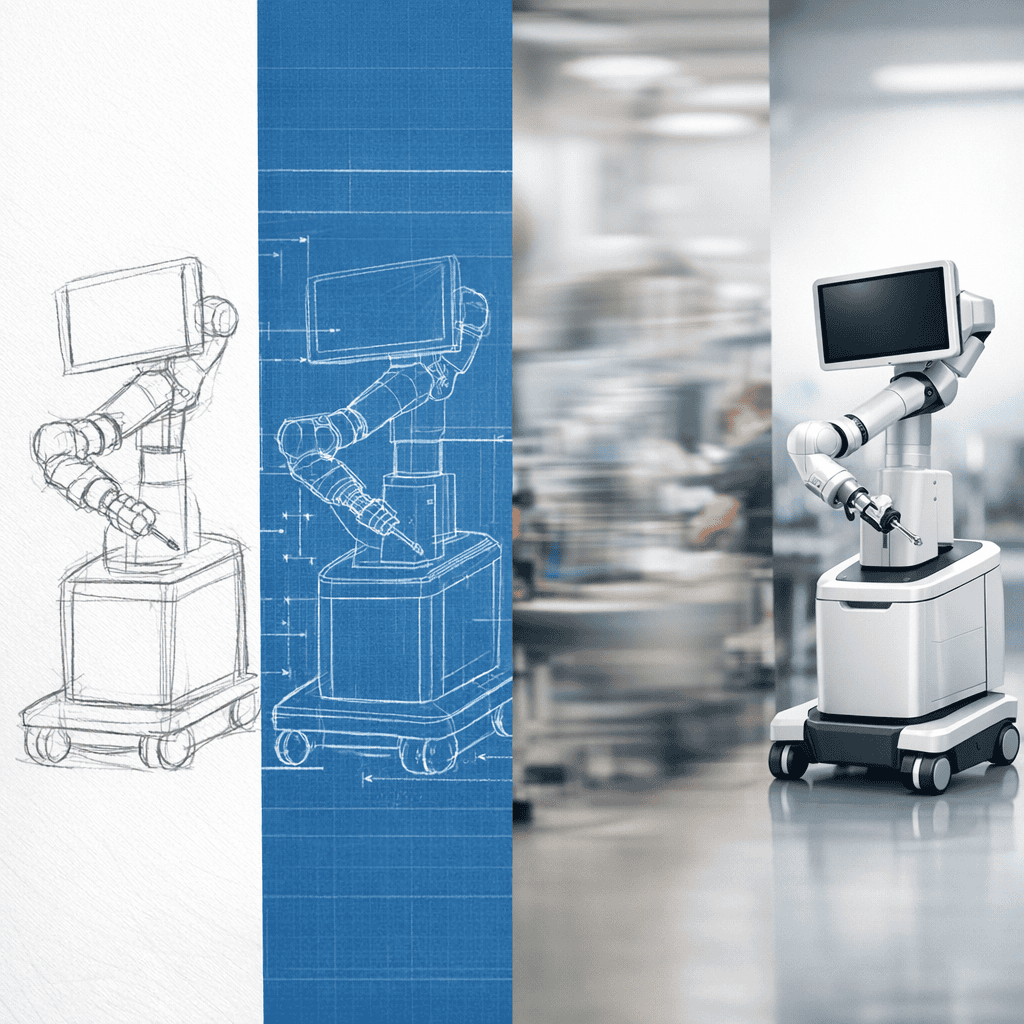
Identify unmet clinical needs and translate them into user-centered designs that improve patient outcomes and clinical efficiency.
our expertise
Here to help with everything from small updates to full-scale redesigns, tailored to your evolving needs.
Quality is Not a Department, It’s a Standard.
Precision at the Speed of Innovation.
Navigating the Path of Least Resistance.
Protecting the Product from Dock to Door.
Engineering for the End-Game.
CDMO, QMS Implementation & Project Management
Project Development & Precision Manufacturing
Comprehensive Regulatory Strategy
Supply Chain & Logistics
Comprehensive Regulatory Strategy
get in touch
Looking to book a consultation or just want to ask a few questions, we’d be glad to connect with you.

Head Engineer | Co-Founder
Eric Klumper
Mailing Address
Please let us know how we can help and we'll be back with you shortly