Boulder Solution

Eric Klumper
Cutting edge med device design. No Bloat.
We bridge the gap between clinical need and commercial reality with absolute regulatory integrity.
End to-end contract design and manufacturing (CDMO) for the risk-averse.
Our approach is straightforward— We engineer bold, defensible regulatory strategies that push your device through the FDA and into the field.
25 +
Explore how we transform ideas into extraordinary digital experiences.
1 + + products in the market

“We combine cutting edge design with precision engineering while -keeping both perfectly aligned with regulatory strategy – getting your project to make it 20% faster.”
Kelsey Lee RAC- Devices Co-Founder

Company expertise
Comprehensive Regulatory Strategy
Project Development & Precision Manufacturing
QMS Implementation & Project Management
Regulatory & Quality Management System (QMS) Consulting
ISO 13485:2016 CERTIFIED | FDA REGISTERED | 21 CFR PART 820 COMPLIANT | ISO CLASS 7 CLEANROOMS
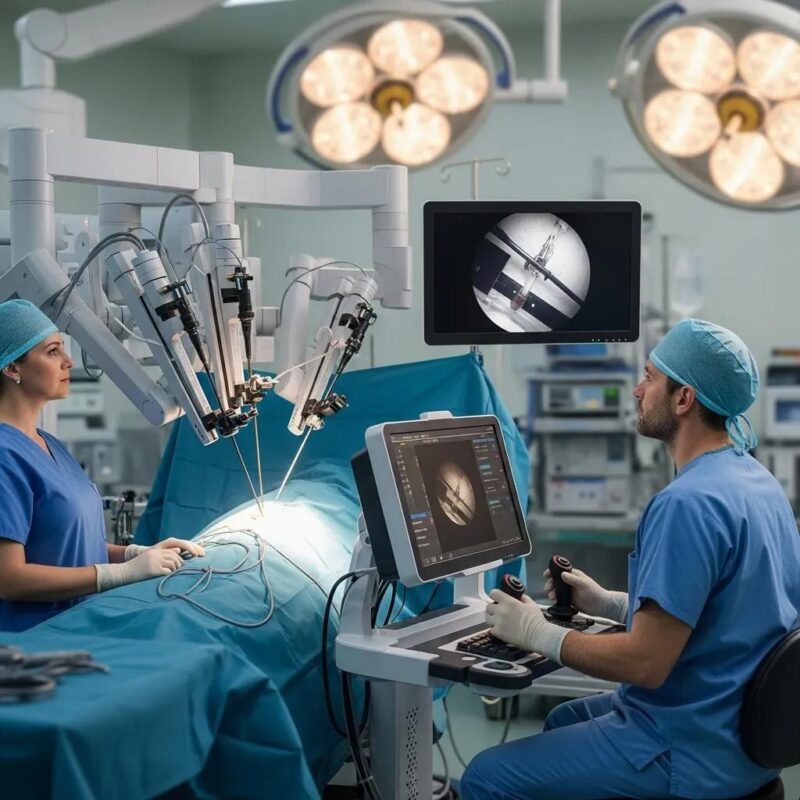
Consistently delivering impactful results through a perfect blend of design and functionality.
110
+
Successful projects in the market


More than 125+ projects completed—each designed to deliver real-world results and withstand for years to come.
Worldwide base
around the world
1
+

From the Founder’s Desk
“We don’t just build medical devices; we engineer confidence. Our mission is to bridge the gap between clinical innovation and commercial reality, ensuring no great idea fails due to avoidable manufacturing errors.”

Eric Klumper
Tell us about your project —whether you just put together a wireframe or have a prototype ready to go.
Work and general inquiries +1 303-667-3002
Rocky River, OH 44116, United States
Have a project in mind?
Driven by passion and grounded in expertise, our team turns bold ideas into reality, leading the way in creative innovation.
Meet our team
What began over coffee-fueled brainstorming sessions has grown into a thriving contract medical device manufacturing and consulting practice dedicated to helping get your project to market with minimal stress.
faq & get answer
Technical FAQ
Haven’t found what you’re looking for yet? Feel free to ask anything. Let’s Talk

Do you handle Class III devices?
Can you rescue a stalled project?
What CAD software do you use?

Do you offer sterilization services?